- About
- Advertise / Support
- Contact
- CancerNetwork.com
- TargetedOnc.com
- OncLive.com
- OncNursingNews.com
- Terms & Conditions
- Privacy
- Do Not Sell My Information
© 2024 MJH Life Sciences™ and CURE - Oncology & Cancer News for Patients & Caregivers. All rights reserved.
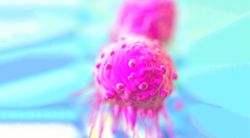
Trial Kicks Off to Study Experimental CAR-T Cell Therapy in Myeloma

Brielle Benyon, Assistant Managing Editor for CURE®, has been with MJH Life Sciences since 2016. She has served as an editor on both CURE and its sister publication, Oncology Nursing News. Brielle is a graduate from The College of New Jersey. Outside of work, she enjoys spending time with family and friends, CrossFit and wishing she had the grace and confidence of her toddler-aged daughter.
The hope, according to an expert, is that this trial will expand CAR-T cell therapy access to more patients with multiple myeloma.
The first patient received treatment in the phase 1 ACLX-001 trial evaluating the novel CAR-T cell therapy, ARC-SparX, in patients with relapsed or refractory multiple myeloma.
The therapy separately uses ARC-T cells and the SparX protein which targets BCMA, a molecule on myeloma cells. ARC-T and SparX come together via a new synthetic binding mechanism called the D-Domain.
In giving the two molecules separately, clinicians can hopefully better control the dosing and frequency of therapy, as well as better manage side effects that typically come along with CAR-T cell therapy.
READ MORE: CAR-T Cell Therapy May Have ‘Big Future’ in Relapsed/ Refractory Multiple Myeloma
"Our ARC-SparX platform, powered by our proprietary D-Domain technology, has the potential to yield transformative therapies that can unleash the full potential of CAR Ts to treat challenging conditions, including solid tumors,” said Rami Elghandour, chairman and CEO of Arcellx, the pharmaceutical company developing the therapy, in a press release “By addressing antigen heterogeneity and dose limiting toxicities, ARC-SparX could help many patients and address significant unmet clinical needs.”.
ACLX-001 is a first-in-human, open-label (meaning that patients and clinicians know what therapy is being administered) dose-escalation trial. The study’s main goal is to determine if ARC-SparX is safe and tolerable. The secondary goal is to determine a dosing schedule.
“We are excited to be participating in this clinical trial to evaluate ACLX-001 in patients with relapsed or refractory multiple myeloma,” said Dr. Binod Dhakal, an associate professor of medicine in the Division of Hematology/Oncology at Medical College of Wisconsin in Milwaukee. “ARC-SparX provides physicians with the ability to control the dose and frequency of SparX administration. This may allow the physician to better manage toxicities associated with traditional CAR T therapies, potentially increasing patient access to this treatment option.”
Of note, Dhakal is an investigator on the phase 1 CART-ddBCMA and ACLX-001 trial.
For more news on cancer updates, research and education, don’t forget to subscribe to CURE®’s newsletters here.
Related Content: